Abstract
Introduction
Hematopoietic stem cell transplant (HSCT) is commonly performed for the treatment of advanced hematological malignancies. Emerging data suggest non-relapse related events, including cardiac arrhythmias, increasingly limit anticancer outcomes and non-relapse mortality after initially successful HSCT. Yet, the relationship between HSCT and increased risk of arrhythmias after transplant remains controversial and unclear. Our aim was to evaluate the incidence and effect on mortality risk of arrhythmia development after HSCT.
Materials and methods
Leveraging the PubMed, Embase, Cochrane, Scopus and Clinicaltrials.gov databases, we identified all published studies evaluating the incidence and impacts of early arrhythmias after HSCT from January 1995 to July 2021 using PRISMA guidelines. We included all available observational studies (case-control, and cohort) and clinical trials without restriction of language or country of publication. The primary outcome was incidence of atrial fibrillation (AF) at 1 year of follow-up after HSCT. Secondary outcomes included the incidence of any arrhythmia across follow-up, the incidence of left atrium (LA) dilation, and non-relapse mortality associated with HSCT induced AF. Subgroup analysis based on type of HSCT treatment employed, allogenic (Allo-HSCT) or autologous (Auto-HSCT), was also performed. Variance weighted random effects modeling (DerSimonian and Laird) was used to define the associations between HSCT and AF and mortality events. Outcomes were reported as event rates, and relative risks (RR). Medians were reported with corresponding standard errors (SE). Heterogeneity was assessed using Cochrane Q-statistic which was quantified with I 2test (>75% was considered high heterogeneity). Publication bias was assessed using Eager's test where applicable. Further, the quality of included studies was assessed using the New-Castle Ottawa scale.
Results
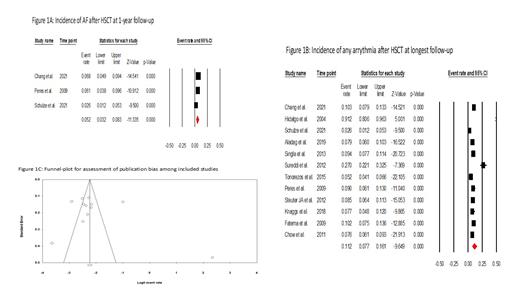
Overall, from 769 articles, 12 cohort studies inclusive of 6,371 patients meeting study-criteria were identified. The mean incidence of AF was 8.8% (I 2:97%, P<0.001), including 5.2% (I 2:65%, P=0.05) within 1-year of any HSCT. The overall incidence of any type of arrhythmia was 11.2% (I 2 =95%, P≤0.001); Figure. Median time to AF onset was 10.3 days (SE= 1.2), but was longer in those treated with Allo-HSCT, at 71 days (SE=68.4); P<0.05. Among those treated with Allo-HSCT, 8.2% (I 2:73%, P<0.001) developed AF, including 6.5% (I 2:72%, P<0.01) at 1 year; the event rate for any type of arrhythmia was 8.3% (I 2:52%, P<0.001). In those treated with Auto-HSCT, the rate of AF was 8.7% (I 2=97%, P≤0.001) across follow-up. Among those with AF following HSCT therapy, LA dilatation was observed in 37.2% (I 2 =61.5%, P=0.1) among available studies. Similarly, mortality was higher among patients who developed AF vs. those without AF after HSCT (RR: 7.4, P=0.008, I 2 = 92.87). There was low risk of publication bias as assessed by visual inspection of funnel plot and Egger's regression test (P= 0.21); and low risk of bias within the included studies.
Conclusion
Atrial fibrillation is increasingly common after HSCT therapy, and associates with increased mortality. The presence of LA remodeling, reflected by atrial dilation, appears to portend AF risk. Further research into the mechanisms and predictors of AF after HSCT are needed.
Vasu: Kiadis, Inc.: Research Funding; Boehringer Ingelheim: Other: Travel support; Seattle Genetics: Other: travel support; Omeros, Inc.: Membership on an entity's Board of Directors or advisory committees. Saad: Incyte Pharmaceuticals: Consultancy; careDx: Consultancy; Amgen: Research Funding; Kadmon: Research Funding; OrcaBio: Research Funding; Magenta Therapeutics: Consultancy. de Lima: Miltenyi Biotec: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees. Jaglowski: Juno: Consultancy; Novartis: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Takeda: Consultancy; CRISPR Therapeutics: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal